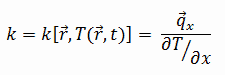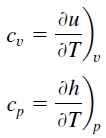About Carbon Dioxide
Carbon dioxide is a colorless gas with a density about 53% higher than that of dry air. It is relatively nontoxic and noncombustible, but it is heavier than air and may asphyxiate by the displacement of air. When CO2 is solved in water, the mild carbonic acid, is formed. Cooled CO2 in solid form is called dry ice. Carbon dioxide is a minor component of Earth’s atmosphere but important constituent of air. It is a necessary raw material for most plant life, which remove carbon dioxide from air using the process of photosynthesis. A typical concentration of CO2 in air is currently about 0.040% or 404 ppm. The concentration of atmospheric carbon dioxide rises and falls in a seasonal pattern over a range of about 6 ppmv. The concentration of CO2 in air has also been steadily increasing from year to year for over 70 years. The current rate of increase is about 2.5 ppm per year.
Summary
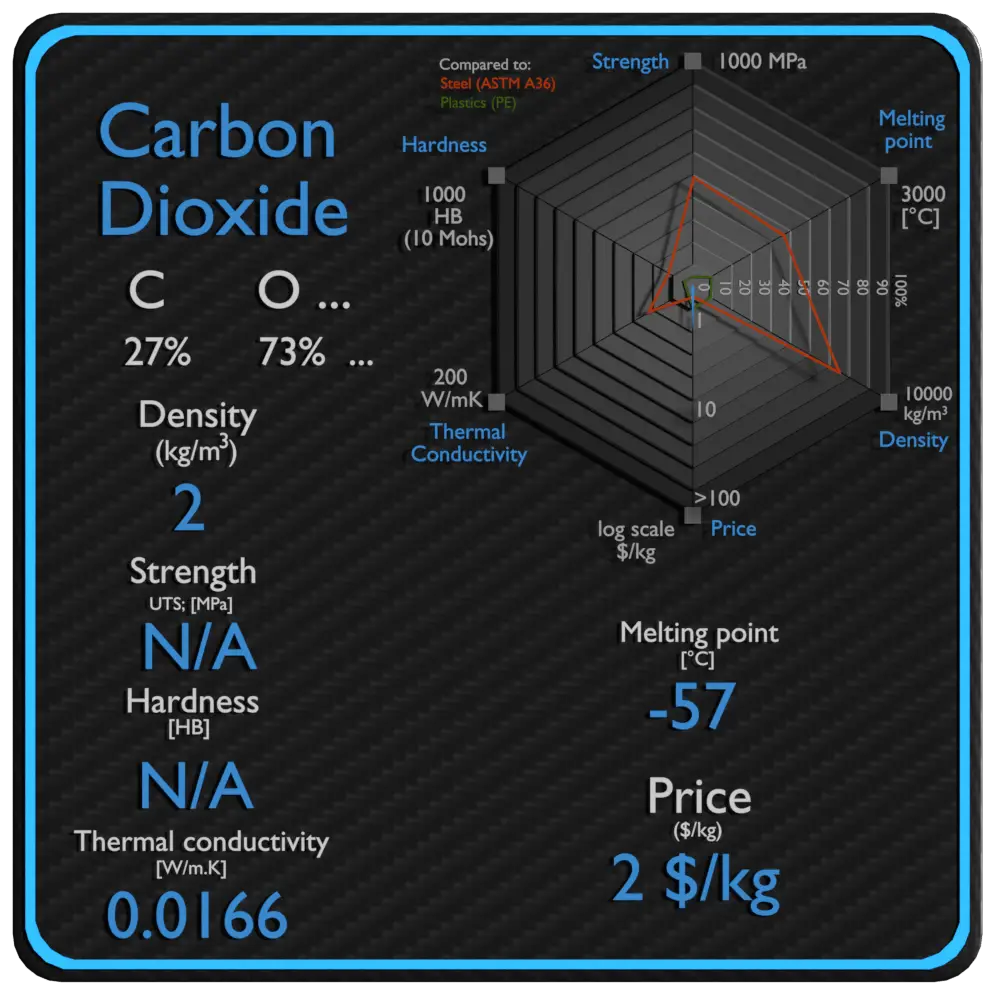
| Name | Carbon Dioxide |
| Phase at STP | gaseous |
| Density | 2 kg/m3 |
| Ultimate Tensile Strength | N/A |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | N/A |
| Brinell Hardness | N/A |
| Melting Point | -57 °C |
| Thermal Conductivity | 0.0166 W/mK |
| Heat Capacity | 840 J/g K |
| Price | 2 $/kg |
Composition of Carbon Dioxide
Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms.
Applications of Carbon Dioxide

Carbon dioxide is used by the food industry, the oil industry, and the chemical industry. The compound has varied commercial uses but one of its greatest uses as a chemical is in the production of carbonated beverages; it provides the sparkle in carbonated beverages such as soda water, beer and sparkling wine. Carbon dioxide also is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses. Carbon dioxide is also used as an atmosphere for welding, although in the welding arc, it reacts to oxidize most metals. Use in the automotive industry is common despite significant evidence that welds made in carbon dioxide are more brittle than those made in more inert atmospheres.
Thermal Properties of Carbon Dioxide
Carbon Dioxide – Melting Point
Melting point of Carbon Dioxide is -57 °C.
Note that, these points are associated with the standard atmospheric pressure. In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. For various chemical compounds and alloys, it is difficult to define the melting point, since they are usually a mixture of various chemical elements.
Carbon Dioxide – Thermal Conductivity
Thermal conductivity of Carbon Dioxide is 0.0166 W/(m·K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
Carbon Dioxide – Specific Heat
Specific heat of Carbon Dioxide is 840 J/g K.
Specific heat, or specific heat capacity, is a property related to internal energy that is very important in thermodynamics. The intensive properties cv and cp are defined for pure, simple compressible substances as partial derivatives of the internal energy u(T, v) and enthalpy h(T, p), respectively:
where the subscripts v and p denote the variables held fixed during differentiation. The properties cv and cp are referred to as specific heats (or heat capacities) because under certain special conditions they relate the temperature change of a system to the amount of energy added by heat transfer. Their SI units are J/kg K or J/mol K.
Properties and prices of other materials
material-table-in-8k-resolution