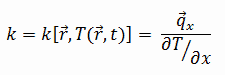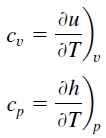About Gasoline
Gasoline, or petrol is a clear petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines. Currently, the main source of fuel for road vehicles is petroleum oil. Oil is a naturally occurring, fossil liquid comprising a complex mixture of hydrocarbons of various molecular weights together with various other organic compounds. These components are separated by fractional distillation in an oil refinery in order to produce gasoline, diesel fuel, kerosene and other products.
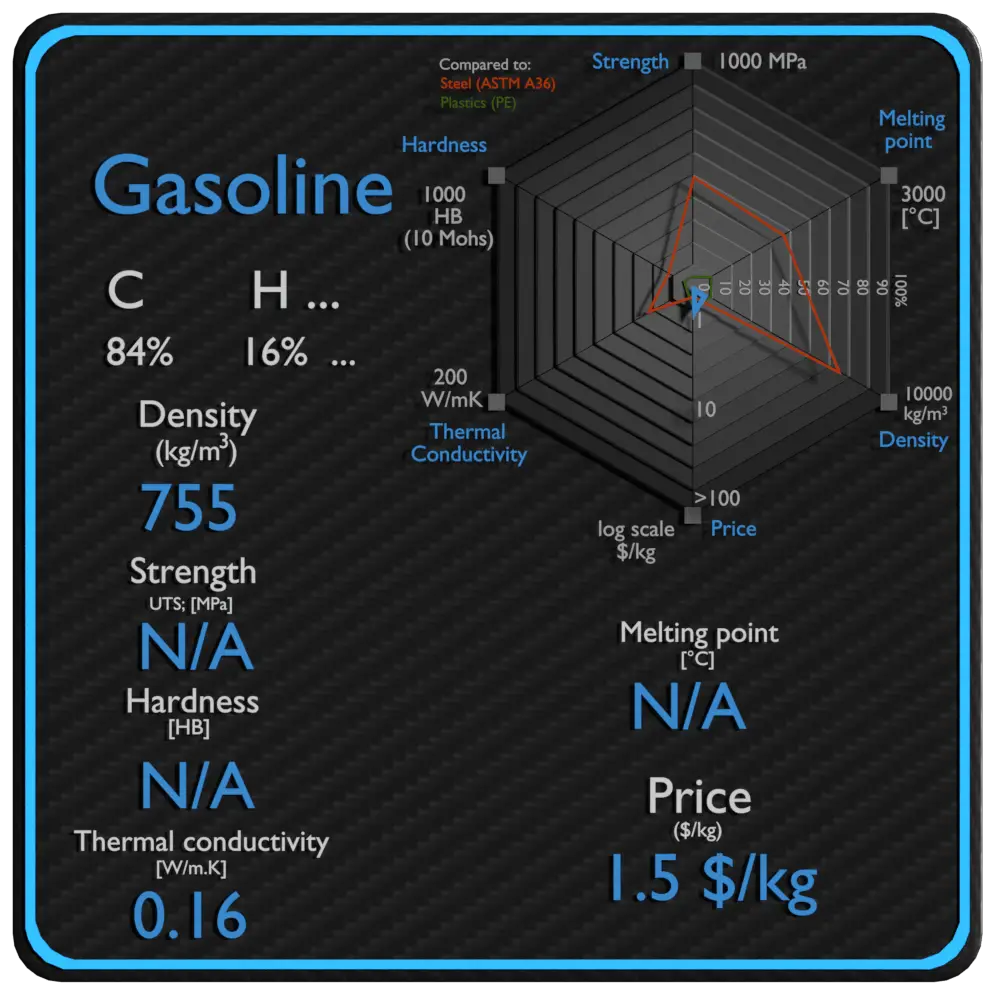
Summary
| Name | Gasoline |
| Phase at STP | liguid |
| Density | 755 kg/m3 |
| Ultimate Tensile Strength | N/A |
| Yield Strength | N/A |
| Young’s Modulus of Elasticity | N/A |
| Brinell Hardness | N/A |
| Melting Point | N/A |
| Thermal Conductivity | 0.16 W/mK |
| Heat Capacity | 2200 J/g K |
| Price | 1.5 $/kg |
Composition of Gasoline
Gasoline, or petroleum spirit (petrol), is a complex mixture consisting of mainly aliphatic hydrocarbons [C5-C13 n-alkanes (17.7%) and C4-C13 branched alkanes (32%), C6-C8 cycloalkanes (5%), C6 olefins (1.8%), aromatics (benzene, toluene, xylene, ethylbenzene, C3-benzenes, C4-benzenes (30.5%)] and other possible components such as octane enhancers (MTBE, TBA, ethanol, methanol), antioxidants, metal deactivators, ignition controllers, icing inhibitors, detergents and corrosion inhibitors.
Applications of Gasoline

Gasoline is used primarily as a fuel in most spark-ignited internal combustion engines. The usage and pricing of gasoline (or petrol) results from factors such as crude oil prices, processing and distribution costs, local demand, the strength of local currencies, local taxation, and the availability of local sources of gasoline (supply). Since fuels are traded worldwide, the trade prices are similar. The price paid by consumers largely reflects national pricing policy. Some regions, such as Europe and Japan, impose high taxes on gasoline (petrol); others, such as Saudi Arabia and Venezuela, subsidize the cost.
Thermal Properties of Gasoline
Gasoline – Melting Point
Melting point of Gasoline is N/A.
Note that, these points are associated with the standard atmospheric pressure. In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium. For various chemical compounds and alloys, it is difficult to define the melting point, since they are usually a mixture of various chemical elements.
Gasoline – Thermal Conductivity
Thermal conductivity of Gasoline is 0.16 W/(m·K).
The heat transfer characteristics of a solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies for all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.
Gasoline – Specific Heat
Specific heat of Gasoline is 2200 J/g K.
Specific heat, or specific heat capacity, is a property related to internal energy that is very important in thermodynamics. The intensive properties cv and cp are defined for pure, simple compressible substances as partial derivatives of the internal energy u(T, v) and enthalpy h(T, p), respectively:
where the subscripts v and p denote the variables held fixed during differentiation. The properties cv and cp are referred to as specific heats (or heat capacities) because under certain special conditions they relate the temperature change of a system to the amount of energy added by heat transfer. Their SI units are J/kg K or J/mol K.
Properties and prices of other materials
material-table-in-8k-resolution