This article contains comparison of key thermal and atomic properties of nickel and tin, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Nickel vs Tin.
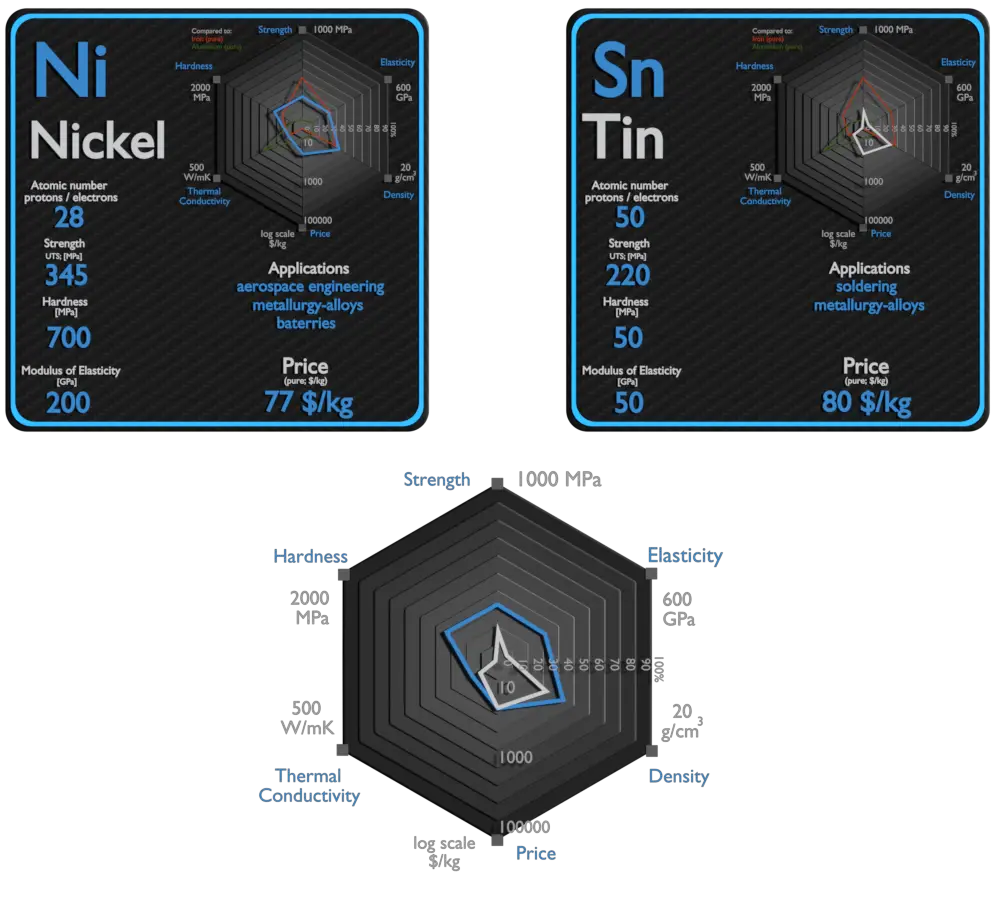
Nickel and Tin – About Elements


Source: www.luciteria.com
Nickel and Tin – Applications
Nickel
The global production of nickel is presently used as follows: 68% in stainless steel; 10% in nonferrous alloys; 9% in electroplating; 7% in alloy steel; 3% in foundries; and 4% other uses (including batteries). Nickel is used as a constituent of different types of alloys; for instance, Monel (corrosion resistant material), Nichrome (an alloy used for resistance heating elements), Permalloy (an alloy with high magnetic permeability at low field strength and low hysteresis loss), cupro-nickel, stainless steel, nickel silver, etc. Nickel based alloys (e.g. Fe-Cr-Ni(Mo) alloys) alloys exhibit excellent ductility and toughness, even at high strength levels and these properties are retained up to low temperatures. Nickel and its alloys are highly resistant to corrosion in many environments, especially those that are basic (alkaline). Nickel also reduces thermal expansion for better dimensional stability. Nickel is the base element for superalloys. These metals have excellent resistance to thermal creep deformation and retain their stiffness, strength, toughness and dimensional stability at temperatures much higher than the other aerospace structural materials.
Tin
The largest single application of tin is in the manufacture of tinplate (steel sheet coated with tin), which accounts for approximately 40% of total world tin consumption. Tin bonds readily to iron and steel to prevent corrosion. Tin-plated steel containers are widely used for food preservation, and this forms a large part of the market for metallic tin. Tinning is the process of thinly coating sheets of wrought iron or steel with tin, and the resulting product is known as tinplate. The term is also widely used for the different process of coating a metal with solder before soldering. There are two processes for the tinning of the black plates: hot-dipping and electroplating.
Nickel and Tin – Comparison in Table
| Element | Nickel | Tin |
| Density | 8.908 g/cm3 | 7.31 g/cm3 |
| Ultimate Tensile Strength | 345 MPa | 220 MPa |
| Yield Strength | 700 MPa | N/A |
| Young’s Modulus of Elasticity | 200 GPa | 50 GPa |
| Mohs Scale | 4 | 1.65 |
| Brinell Hardness | 700 MPa | 50 MPa |
| Vickers Hardness | 640 MPa | N/A |
| Melting Point | 1455 °C | 231.93 °C |
| Boiling Point | 2730 °C | 2602 °C |
| Thermal Conductivity | 90.7 W/mK | 67 W/mK |
| Thermal Expansion Coefficient | 13.4 µm/mK | 22 µm/mK |
| Specific Heat | 0.44 J/g K | 0.227 J/g K |
| Heat of Fusion | 17.47 kJ/mol | 7.029 kJ/mol |
| Heat of Vaporization | 370.4 kJ/mol | 295.8 kJ/mol |










