This article contains comparison of key thermal and atomic properties of potassium and calcium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Potassium vs Calcium.
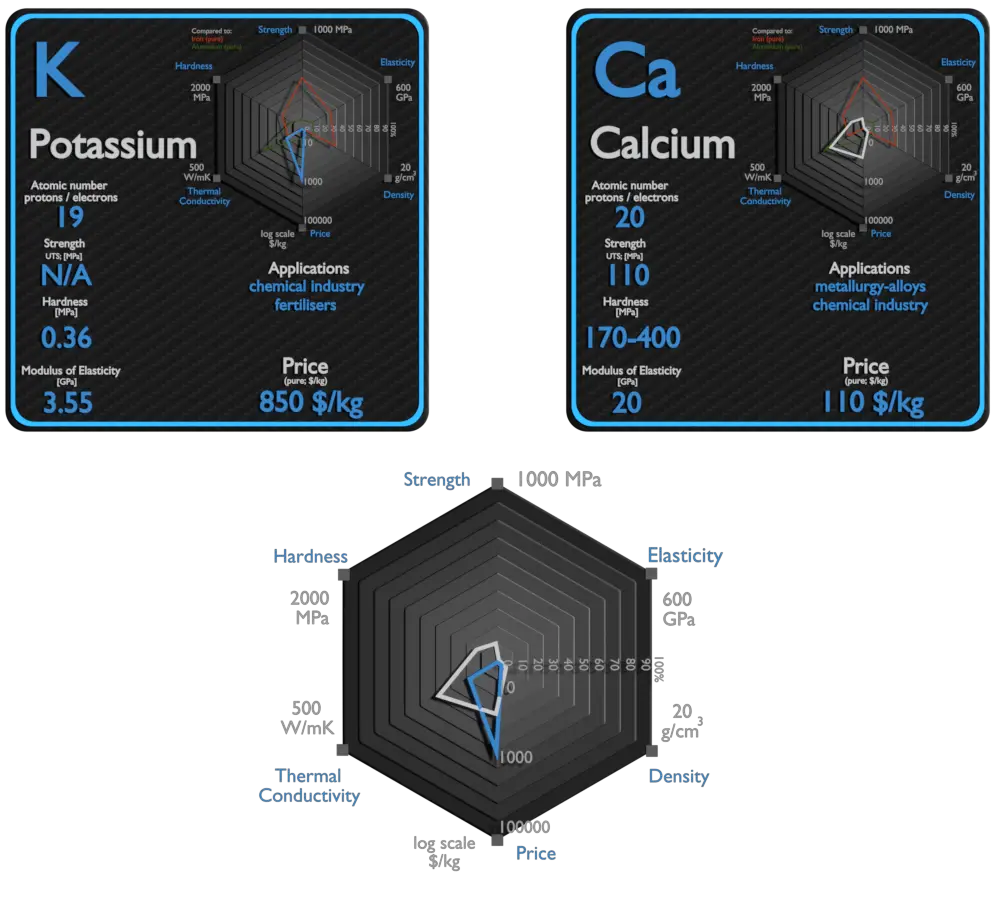
Potassium and Calcium – About Elements

Source: www.luciteria.com
Potassium and Calcium – Applications
Potassium
Potassium (K) is an essential nutrient for plant growth. It’s classified as a macronutrient because plants take up large quantities of K during their life cycle. Agricultural fertilizers consume 95% of global potassium chemical production, and about 90% of this potassium is supplied as KCl. Due to its high degree of reactivity, pure potassium is rarely used in its elemental /metallic form. It is used as a powerful reducing agent in organic chemistry. Potassium/Sodium alloys are It used as a heat exchange medium . The heat in the potassium warms water and makes it hot enough to boil. Then water is changed into steam, which is used to work devices that generate electricity.
Calcium
The largest use of metallic calcium is in steelmaking, due to its strong chemical affinity for oxygen and sulfur. Its oxides and sulfides, once formed, give liquid lime aluminate and sulfide inclusions in steel which float out. Calcium compounds are used as manufacture of insecticides, paints, blackboard chalk, textile and fireworks.
Potassium and Calcium – Comparison in Table
| Element | Potassium | Calcium |
| Density | 0.856 g/cm3 | 1.55 g/cm3 |
| Ultimate Tensile Strength | N/A | 110 MPa |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | 3.53 GPa | 20 GPa |
| Mohs Scale | 0.4 | 1.5 |
| Brinell Hardness | 0.36 MPa | 170 – 400 MPa |
| Vickers Hardness | N/A | N/A |
| Melting Point | 63.25 °C | 842 °C |
| Boiling Point | 760 °C | 1484 °C |
| Thermal Conductivity | 102.4 W/mK | 200 W/mK |
| Thermal Expansion Coefficient | 83 µm/mK | 22.3 µm/mK |
| Specific Heat | 0.75 J/g K | 0.63 J/g K |
| Heat of Fusion | 2.334 kJ/mol | 8.54 kJ/mol |
| Heat of Vaporization | 79.87 kJ/mol | 153.3 kJ/mol |