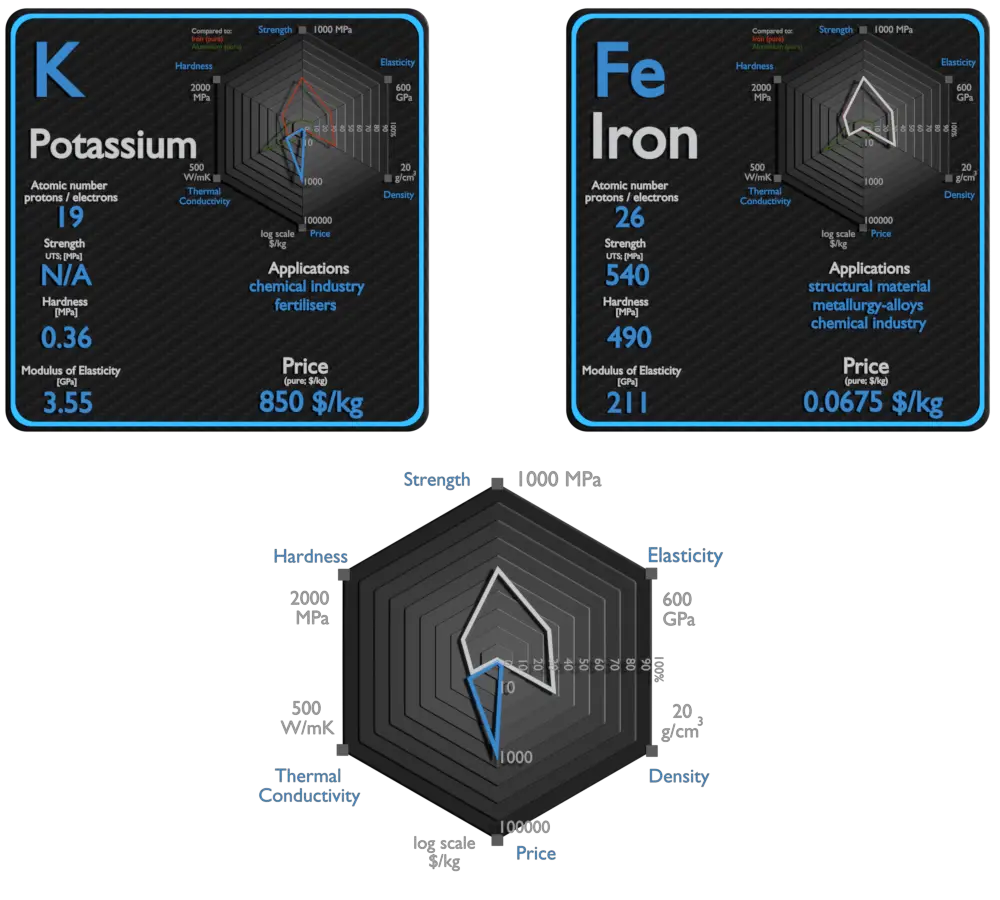
This article contains comparison of key thermal and atomic properties of potassium and iron, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Potassium vs Iron.
Potassium and Iron – About Elements

Source: www.luciteria.com
Potassium and Iron – Applications
Potassium
Potassium (K) is an essential nutrient for plant growth. It’s classified as a macronutrient because plants take up large quantities of K during their life cycle. Agricultural fertilizers consume 95% of global potassium chemical production, and about 90% of this potassium is supplied as KCl. Due to its high degree of reactivity, pure potassium is rarely used in its elemental /metallic form. It is used as a powerful reducing agent in organic chemistry. Potassium/Sodium alloys are It used as a heat exchange medium . The heat in the potassium warms water and makes it hot enough to boil. Then water is changed into steam, which is used to work devices that generate electricity.
Iron
Iron is used in numerous sectors such as electronics, manufacturing, automotive, and construction and building. Iron is the most widely used of all the metals, accounting for over 90% of worldwide metal produc0tion. Its low cost and high strength often make it the material of choice material to withstand stress or transmit forces, such as the construction of machinery and machine tools, rails, automobiles, ship hulls, concrete reinforcing bars, and the load-carrying framework of buildings. Since pure iron is quite soft, it is most commonly combined with alloying elements to make steel. Steels are iron–carbon alloys that may contain appreciable concentrations of other alloying elements. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Due to its very-high strength, but still substantial toughness, and its ability to be greatly altered by heat treatment, steel is one of the most useful and common ferrous alloy in modern use. There are thousands of alloys that have different compositions and/or heat treatments. The mechanical properties are sensitive to the content of carbon, which is normally less than 1.0 wt%.
Potassium and Iron – Comparison in Table
| Element | Potassium | Iron |
| Density | 0.856 g/cm3 | 7.874 g/cm3 |
| Ultimate Tensile Strength | N/A | 540 MPa |
| Yield Strength | N/A | 50 MPa |
| Young’s Modulus of Elasticity | 3.53 GPa | 211 GPa |
| Mohs Scale | 0.4 | 4.5 |
| Brinell Hardness | 0.36 MPa | 490 MPa |
| Vickers Hardness | N/A | 608 MPa |
| Melting Point | 63.25 °C | 1538 °C |
| Boiling Point | 760 °C | 2861 °C |
| Thermal Conductivity | 102.4 W/mK | 80.2 W/mK |
| Thermal Expansion Coefficient | 83 µm/mK | 11.8 µm/mK |
| Specific Heat | 0.75 J/g K | 0.44 J/g K |
| Heat of Fusion | 2.334 kJ/mol | 13.8 kJ/mol |
| Heat of Vaporization | 79.87 kJ/mol | 349.6 kJ/mol |