This article contains comparison of key thermal and atomic properties of aluminium and copper, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Aluminium vs Copper.
Aluminium and Copper – About Elements


Source: www.luciteria.com
Aluminium and Copper – Applications
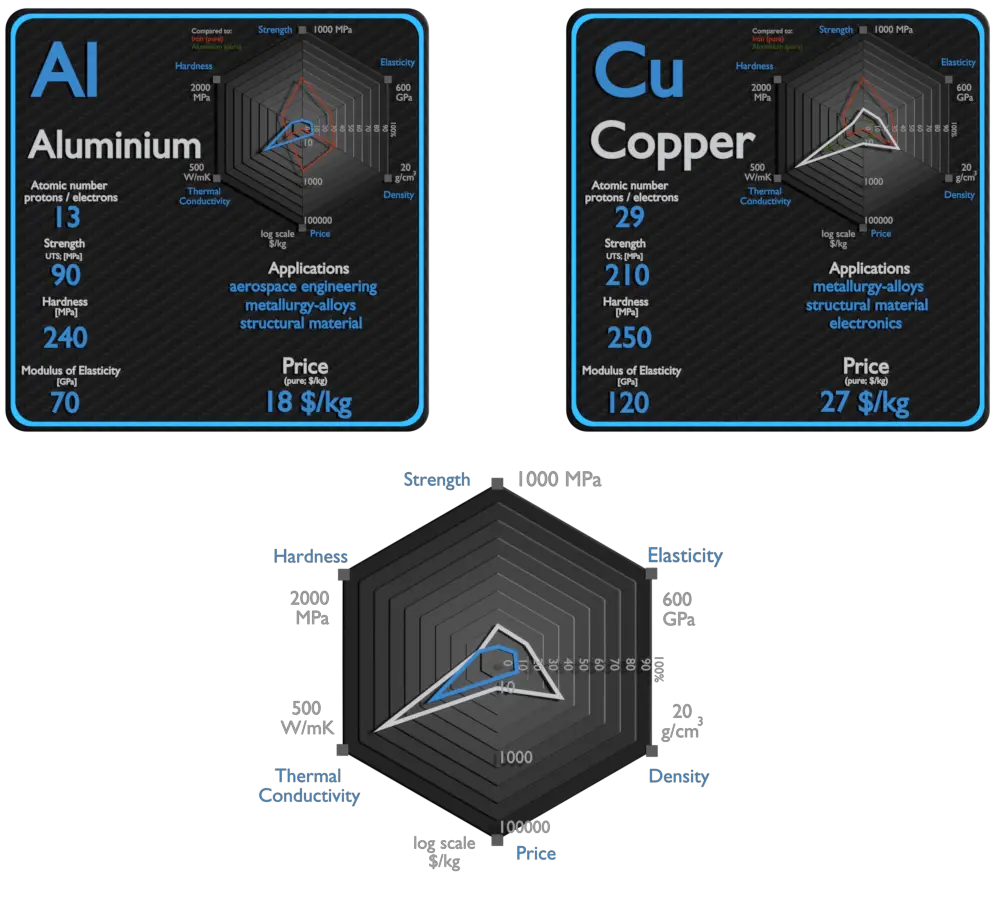
Aluminium
Aluminium and its alloys are used widely in aerospace, automotive, architectural, lithographic, packaging, electrical and electronic applications. It is the prime material of construction for the aircraft industry throughout most of its history. About 70% of commercial civil aircraft airframes are made from aluminium alloys, and without aluminium civil aviation would not be economically viable. Automotive industry now includes aluminium as engine castings, wheels, radiators and increasingly as body parts. 6111 aluminium and 2008 aluminium alloy are extensively used for external automotive body panels. Cylinder blocks and crankcases are often cast made of aluminium alloys.
Copper
Historically, alloying copper with another metal, for example tin to make bronze, was first practiced about 4000 years after the discovery of copper smelting, and about 2000 years after “natural bronze” had come into general use. An ancient civilization is defined to be in the Bronze Age either by producing bronze by smelting its own copper and alloying with tin, arsenic, or other metals. The major applications of copper are electrical wire (60%), roofing and plumbing (20%), and industrial machinery (15%). Copper is used mostly as a pure metal, but when greater hardness is required, it is put into such alloys as brass and bronze (5% of total use). Copper and copper-based alloys including brasses (Cu-Zn) and bronzes (Cu-Sn) are widely used in different industrial and societal applications. Some of the common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Bronze, or bronze-like alloys and mixtures, were used for coins over a longer period. is still widely used today for springs, bearings, bushings, automobile transmission pilot bearings, and similar fittings, and is particularly common in the bearings of small electric motors. Brass and bronze are common engineering materials in modern architecture and primarily used for roofing and facade cladding due to their visual appearance.
Aluminium and Copper – Comparison in Table
| Element | Aluminium | Copper |
| Density | 2.7 g/cm3 | 8.92 g/cm3 |
| Ultimate Tensile Strength | 90 MPa (pure), 600 MPa (alloys) | 210 MPa |
| Yield Strength | 11 MPa (pure), 400 MPa (alloys) | 33 MPa |
| Young’s Modulus of Elasticity | 70 GPa | 120 GPa |
| Mohs Scale | 2.8 | 3 |
| Brinell Hardness | 240 MPa | 250 MPa |
| Vickers Hardness | 167 MPa | 350 MPa |
| Melting Point | 660 °C | 1084.62 °C |
| Boiling Point | 2467 °C | 2562 °C |
| Thermal Conductivity | 237 W/mK | 401 W/mK |
| Thermal Expansion Coefficient | 23.1 µm/mK | 16.5 µm/mK |
| Specific Heat | 0.9 J/g K | 0.38 J/g K |
| Heat of Fusion | 10.79 kJ/mol | 13.05 kJ/mol |
| Heat of Vaporization | 293.4 kJ/mol | 300.3 kJ/mol |