This article contains comparison of key thermal and atomic properties of hydrogen and carbon, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Hydrogen vs Carbon.
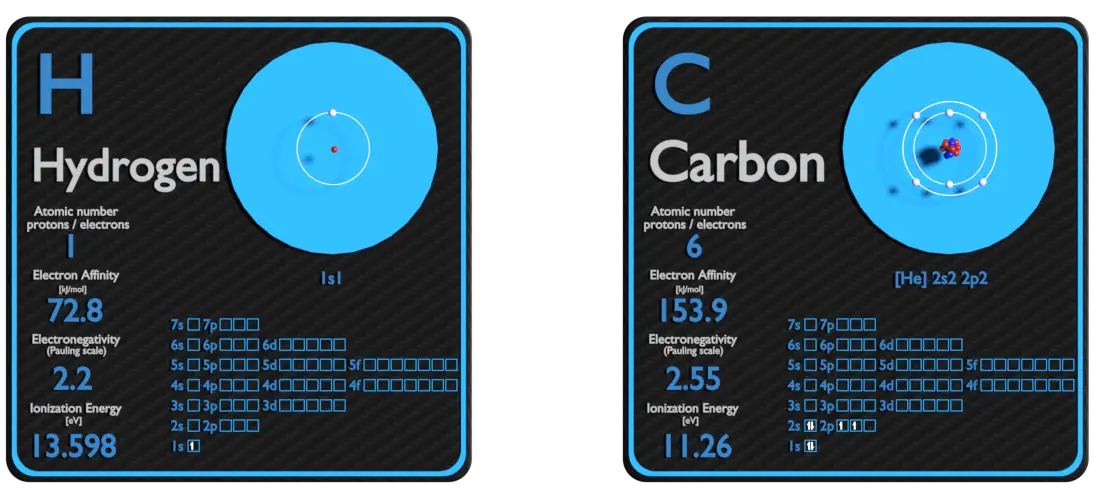
Hydrogen and Carbon – About Elements


Source: www.luciteria.com
Hydrogen and Carbon – Applications
Hydrogen
Hydrogen is versatile and can be utilized in various ways. These multiple uses can be grouped into two large categories. Hydrogen as a feedstock. A role whose importance is being recognized for decades and will continue to grow and evolve. The largest single use of hydrogen in the world is in ammonia manufacture, which consumes about two-thirds of the world’s hydrogen production. Hydrogen is versatile and can be utilized in various ways. These multiple uses can be grouped into two large categories. Hydrogen as a feedstock for further chemical processes. A role whose importance is being recognized for decades and will continue to grow and evolve. And hydrogen as an energy carrier. Hydrogen is also commonly used in power stations as a coolant in generators due to a number of favorable properties that are a direct result of its light diatomic molecules.
Carbon
The major economic use of carbon other than food and wood is in the form of hydrocarbons, most notably the fossil fuel methane gas and crude oil (petroleum). Graphite and diamonds are two important allotropes of carbon that have wide applications. The uses of carbon and its compounds are extremely varied. It can form alloys with iron, of which the most common is carbon steel. Carbon is a non-metallic element, which is an important alloying element in all ferrous metal based materials. Carbon is always present in metallic alloys, i.e. in all grades of stainless steel and heat resistant alloys. Carbon is a very strong austenitizer and increases the strength of steel. In fact, it is the principal hardening element and is essential to the formation of cementite, Fe3C, pearlite, spheroidite, and iron-carbon martensite. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Graphite is combined with clays to form the ‘lead’ used in pencils used for writing and drawing. It is also used as a lubricant and a pigment, as a molding material in glass manufacture, in electrodes for dry batteries and in electroplating and electroforming, in brushes for electric motors and as a neutron moderator in nuclear reactors. Charcoal has been used since earliest times for a large range of purposes including art and medicine, but by far its most important use has been as a metallurgical fuel. Carbon fibers are used where low weight, high stiffness, high conductivity, or where the look of the carbon fiber weave desired.
Hydrogen and Carbon – Comparison in Table
| Element | Hydrogen | Carbon |
| Density | 0.00009 g/cm3 | 2.26 g/cm3 |
| Ultimate Tensile Strength | N/A | 15 MPa (graphite); 3500 MPa (carbon fiber) |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | N/A | 4.1 GPa (graphite); 228 GPa (carbon fiber) |
| Mohs Scale | N/A | 0.8 (graphite) |
| Brinell Hardness | N/A | N/A |
| Vickers Hardness | N/A | N/A |
| Melting Point | -259.1 °C | 4099 °C |
| Boiling Point | -252.9 °C | 4527 °C |
| Thermal Conductivity | 0.1805 W/mK | 129 W/mK |
| Thermal Expansion Coefficient | — µm/mK | 0.8 µm/mK |
| Specific Heat | 14.304 J/g K | 0.71 J/g K |
| Heat of Fusion | 0.05868 kJ/mol | — kJ/mol |
| Heat of Vaporization | 0.44936 kJ/mol | 355.8 kJ/mol |