This article contains comparison of key thermal and atomic properties of carbon and aluminium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Carbon vs Aluminium.
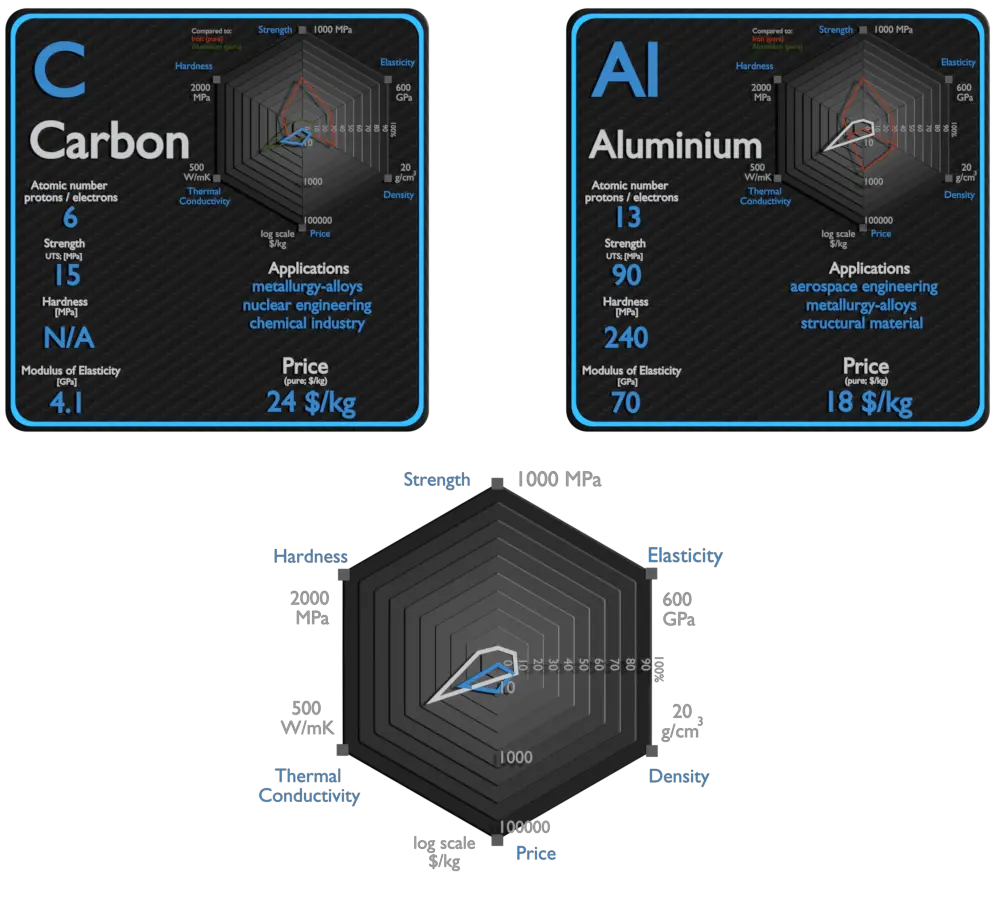
Carbon and Aluminium – About Elements


Source: www.luciteria.com
Carbon and Aluminium – Applications
Carbon
The major economic use of carbon other than food and wood is in the form of hydrocarbons, most notably the fossil fuel methane gas and crude oil (petroleum). Graphite and diamonds are two important allotropes of carbon that have wide applications. The uses of carbon and its compounds are extremely varied. It can form alloys with iron, of which the most common is carbon steel. Carbon is a non-metallic element, which is an important alloying element in all ferrous metal based materials. Carbon is always present in metallic alloys, i.e. in all grades of stainless steel and heat resistant alloys. Carbon is a very strong austenitizer and increases the strength of steel. In fact, it is the principal hardening element and is essential to the formation of cementite, Fe3C, pearlite, spheroidite, and iron-carbon martensite. Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Graphite is combined with clays to form the ‘lead’ used in pencils used for writing and drawing. It is also used as a lubricant and a pigment, as a molding material in glass manufacture, in electrodes for dry batteries and in electroplating and electroforming, in brushes for electric motors and as a neutron moderator in nuclear reactors. Charcoal has been used since earliest times for a large range of purposes including art and medicine, but by far its most important use has been as a metallurgical fuel. Carbon fibers are used where low weight, high stiffness, high conductivity, or where the look of the carbon fiber weave desired.
Aluminium
Aluminium and its alloys are used widely in aerospace, automotive, architectural, lithographic, packaging, electrical and electronic applications. It is the prime material of construction for the aircraft industry throughout most of its history. About 70% of commercial civil aircraft airframes are made from aluminium alloys, and without aluminium civil aviation would not be economically viable. Automotive industry now includes aluminium as engine castings, wheels, radiators and increasingly as body parts. 6111 aluminium and 2008 aluminium alloy are extensively used for external automotive body panels. Cylinder blocks and crankcases are often cast made of aluminium alloys.
Carbon and Aluminium – Comparison in Table
| Element | Carbon | Aluminium |
| Density | 2.26 g/cm3 | 2.7 g/cm3 |
| Ultimate Tensile Strength | 15 MPa (graphite); 3500 MPa (carbon fiber) | 90 MPa (pure), 600 MPa (alloys) |
| Yield Strength | N/A | 11 MPa (pure), 400 MPa (alloys) |
| Young’s Modulus of Elasticity | 4.1 GPa (graphite); 228 GPa (carbon fiber) | 70 GPa |
| Mohs Scale | 0.8 (graphite) | 2.8 |
| Brinell Hardness | N/A | 240 MPa |
| Vickers Hardness | N/A | 167 MPa |
| Melting Point | 4099 °C | 660 °C |
| Boiling Point | 4527 °C | 2467 °C |
| Thermal Conductivity | 129 W/mK | 237 W/mK |
| Thermal Expansion Coefficient | 0.8 µm/mK | 23.1 µm/mK |
| Specific Heat | 0.71 J/g K | 0.9 J/g K |
| Heat of Fusion | N/A | 10.79 kJ/mol |
| Heat of Vaporization | 355.8 kJ/mol | 293.4 kJ/mol |