This article contains comparison of key thermal and atomic properties of sodium and potassium, two comparable chemical elements from the periodic table. It also contains basic descriptions and applications of both elements. Sodium vs Potassium.
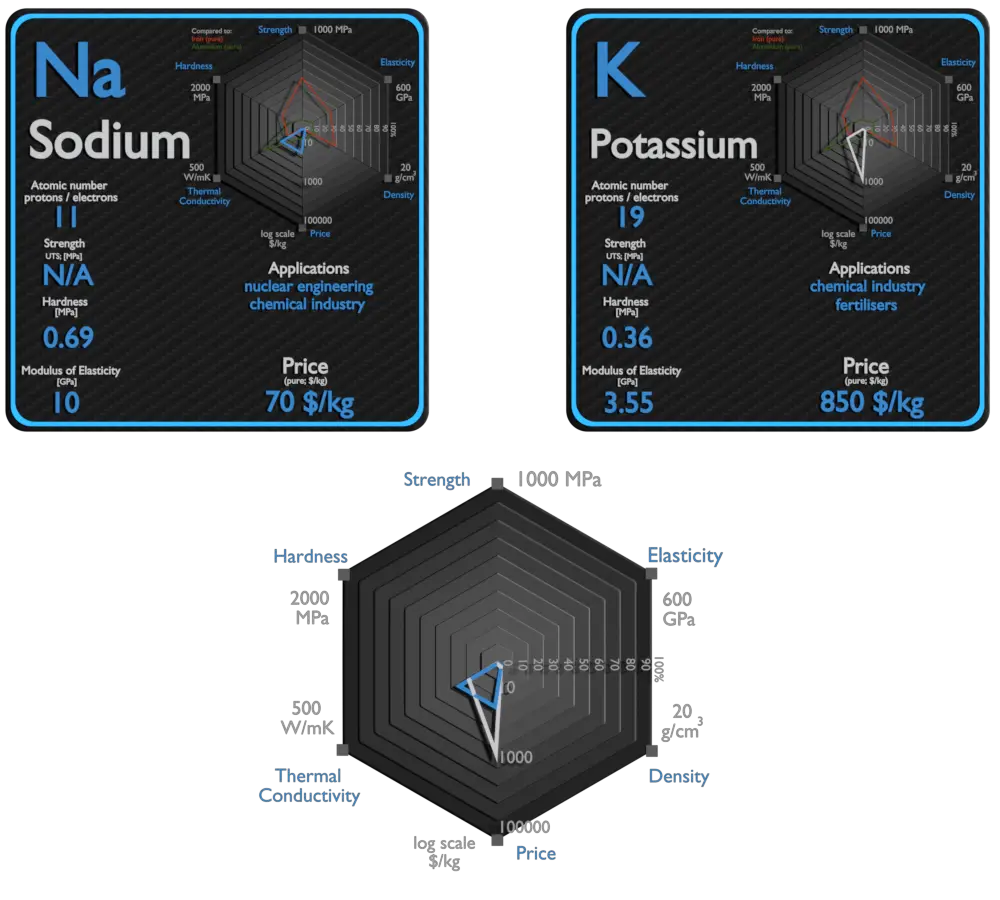
Sodium and Potassium – About Elements

Source: www.luciteria.com
Sodium and Potassium – Applications
Sodium
Metallic sodium is used mainly for the production of sodium borohydride, sodium azide, indigo, and triphenylphosphine. A once-common use was the making of tetraethyllead and titanium metal; because of the move away from TEL and new titanium production methods. An electric current and sodium vapor combine to form a yellowish glow. This principle is used for the making of sodium vapor lamps. Sodium is occasionally used as a heat exchange medium in nuclear power plants. Liquid sodium is sealed into pipes surrounding the reactor core. Generated heat is absorbed by sodium and forced through the pipes in a heat exchanger which can be used to generate electricity.
Potassium
Potassium (K) is an essential nutrient for plant growth. It’s classified as a macronutrient because plants take up large quantities of K during their life cycle. Agricultural fertilizers consume 95% of global potassium chemical production, and about 90% of this potassium is supplied as KCl. Due to its high degree of reactivity, pure potassium is rarely used in its elemental /metallic form. It is used as a powerful reducing agent in organic chemistry. Potassium/Sodium alloys are It used as a heat exchange medium . The heat in the potassium warms water and makes it hot enough to boil. Then water is changed into steam, which is used to work devices that generate electricity.
Sodium and Potassium – Comparison in Table
| Element | Sodium | Potassium |
| Density | 0.968 g/cm3 | 0.856 g/cm3 |
| Ultimate Tensile Strength | N/A | N/A |
| Yield Strength | N/A | N/A |
| Young’s Modulus of Elasticity | 10 GPa | 3.53 GPa |
| Mohs Scale | 0.4 | 0.4 |
| Brinell Hardness | 0.69 MPa | 0.36 MPa |
| Vickers Hardness | N/A | N/A |
| Melting Point | 97.8 °C | 63.25 °C |
| Boiling Point | 883 °C | 760 °C |
| Thermal Conductivity | 141 W/mK | 102.4 W/mK |
| Thermal Expansion Coefficient | 71 µm/mK | 83 µm/mK |
| Specific Heat | 1.23 J/g K | 0.75 J/g K |
| Heat of Fusion | 2.598 kJ/mol | 2.334 kJ/mol |
| Heat of Vaporization | 96.96 kJ/mol | 79.87 kJ/mol |