Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid.
Protons and Neutrons in Arsenic
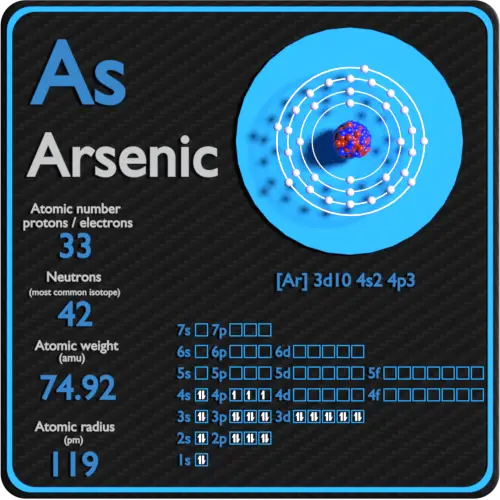
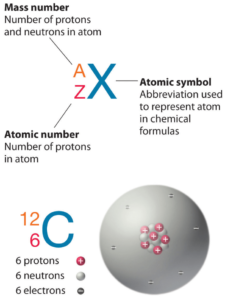 Arsenic is a chemical element with atomic number 33 which means there are 33 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
Arsenic is a chemical element with atomic number 33 which means there are 33 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.
For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Mass numbers of typical isotopes of Arsenic are 75.
Main Isotopes of Arsenic
Arsenic occurs only one natural isotope – 75As.
Arsenic-75 is composed of 33 protons, 42 neutrons, and 33 electrons.
Naturally Occuring Isotopes
| Isotope | Abundance | Neutron Number |
| 75As | 100% | 42 |
Typical Unstable Isotopes
| Isotope | Half-life | Decay Mode | Product |
| 73As | 80.3 d | electron capture | 73Ge |
| 74As | 17.8 d | electron capture | 74Ge |
Electrons and Electron Configuration
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Arsenic is 33. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
Since the number of electrons and their arrangement are responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
Electron configuration of Arsenic is [Ar] 3d10 4s2 4p3.
Possible oxidation states are +3,5/-3.
Most Common Application of Arsenic
The elemental form of arsenic is commonly used in alloys for lead-acid batteries and cable sheaths. Arsenic compounds are also used in semiconductors and light-emitting diodes. Arsenic is used as a doping agent in semiconductors (gallium arsenide) for solid-state devices. It is also used in bronzing, pyrotechnics and for hardening shot.
Summary
| Element | Arsenic |
| Number of protons | 33 |
| Number of neutrons (typical isotopes) | 75 |
| Number of electrons | 33 |
| Electron configuration | [Ar] 3d10 4s2 4p3 |
| Oxidation states | +3,5/-3 |
Source: www.luciteria.com