Nitrogen is a colourless, odourless unreactive gas that forms about 78% of the earth’s atmosphere. Nitrogen in various chemical forms plays a major role in large number of environmental issues.
Two-thirds of nitrogen produced by industry is sold as the gas and the remaining one-third as the liquid. Synthetically produced ammonia and nitrates are key industrial fertilisers, and fertiliser nitrates are key pollutants in the eutrophication of water systems. Apart from its use in fertilisers and energy-stores, nitrogen is a constituent of organic compounds as diverse as Kevlar used in high-strength fabric and cyanoacrylate used in superglue.
Dinitrogen forms about 78% of Earth’s atmosphere, making it the most abundant uncombined element. Nitrogen gas is an industrial gas produced by the fractional distillation of liquid air, or by mechanical means using gaseous air (pressurised reverse osmosis membrane or pressure swing adsorption).
Protons and Neutrons in Nitrogen
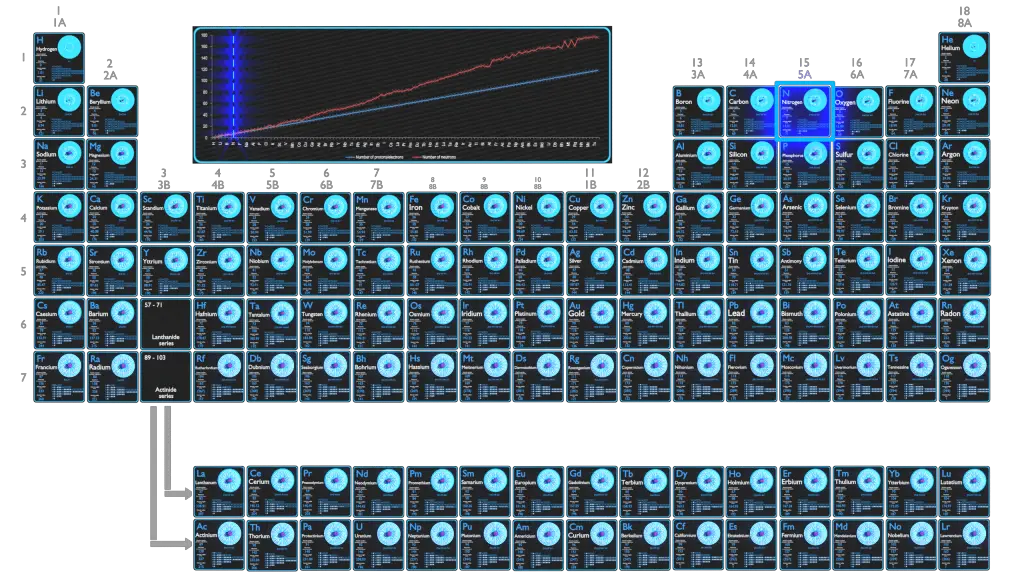
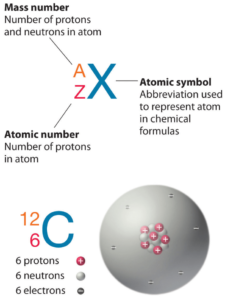 Nitrogen is a chemical element with atomic number 7 which means there are 7 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A. The difference between the neutron number and the atomic number is known as the neutron excess: D = N – Z = A – 2Z.
For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the number of neutrons. Mass numbers of typical isotopes of Nitrogen are 14; 15.
Main Isotopes of Nitrogen
Nitrogen has two stable isotopes: 14N and 15N. The first is much more common, making up 99.634% of natural nitrogen, and the second (which is slightly heavier) makes up the remaining 0.366%. This leads to an atomic weight of around 14.007 u.
Nitrogen-14 is composed of 7 protons, 7 neutrons, and 7 electrons. Nitrogen-14 is one of the very few stable nuclides with both an odd number of protons and of neutrons (seven each) and is the only one to make up a majority of its element.
Nitrogen-15 is composed of 7 protons, 8 neutrons, and 7 electrons. Two sources of nitrogen-15 are the positron emission of oxygen-15 and the beta decay of carbon-15. Nitrogen-15 presents one of the lowest thermal neutron capture cross sections of all isotopes.
Nitrogen-16 is composed of 7 protons, 9 neutrons, and 7 electrons. In nuclear reactors, nitrogen-16 can be used to detect leakages from steam generators. Nitrogen-16 is an isotope of nitrogen generated by neutron activation of oxygen contained in the water. It has a short half-life of 7.1 sec and it decays via beta decay. This decay is accompanied by emission of a very energetic gamma rays (6 MeV), which can readily penetrate the wall of the high-pressure piping and are therefore can be easily measured by ion chambers located on the hot leg piping of each coolant loop.
Stable Isotopes
| Isotope | Abundance | Neutron Number |
| 14N | 99.6% | 7 |
| 15N | 0.4% | 8 |
Typical Unstable Isotopes
| Isotope | Half-life | Decay Mode | Product |
| 13N | 9.965 min | electron capture | 13C |
| 16N | 7.1 s | beta decay | 16O |
Electrons and Electron Configuration
The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Nitrogen is 7. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.
Since the number of electrons and their arrangement are responsible for the chemical behavior of atoms, the atomic number identifies the various chemical elements. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z.
Electron configuration of Nitrogen is [He] 2s2 2p3.
Possible oxidation states are +1,2,3,4,5/-1,2,3.
Many industrially important compounds, such as ammonia, nitric acid, organic nitrates (propellants and explosives), and cyanides, contain nitrogen. The extremely strong triple bond in elemental nitrogen (N≡N), the second strongest bond in any diatomic molecule after carbon monoxide (CO),[3] dominates nitrogen chemistry. This causes difficulty for both organisms and industry in converting N2 into useful compounds, but at the same time means that burning, exploding, or decomposing nitrogen compounds to form nitrogen gas releases large amounts of often useful energy.
Most Common Chemical Compound of Nitrogen
Ammonia is a compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a characteristic pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers.
Summary
| Element | Nitrogen |
| Number of protons | 7 |
| Number of neutrons (typical isotopes) | 14; 15 |
| Number of electrons | 7 |
| Electron configuration | [He] 2s2 2p3 |
| Oxidation states | +1,2,3,4,5/-1,2,3 |
Source: www.luciteria.com